Mass is always conserved in chemical reactions. Steps to Balancing Chemical Equations 1.

Loeblein S Chemistry Phet Clicker Questions Ppt Download
Our products are Fe2 and O 3.
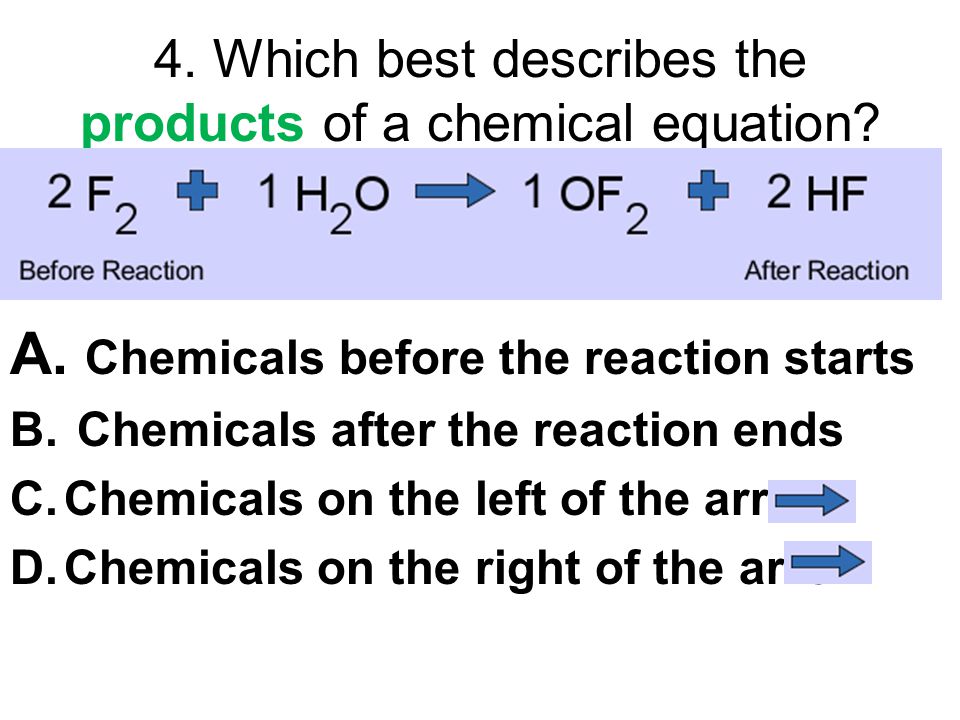
. Coefficients in a chemical equation express ________________ between molecules or compounds. Min is asked to balance the equation below. We can balance this equation using the following steps Step I.
Write the equation by putting the reactants on the left of the arrow and the products on the right. Since Cu and N only occur once on each side of the equation it is wise to balance them first. Which best describes Mins method for balancing the chemical equation.
An equation with equal numbers of each type of atom on both sides of the arrow is called a balanced chemical reactionA written symbolic representation of a chemical reaction is called a chemical equation. When there is no. Thus one of the coefficients is arbitrary and all the others can be expressed relative to it.
How to Balance Chemical Equations. To be useful chemical equations must always be balanced. The equation identifies the reactants starting materials and products resulting substances the formulas of the participants the phases of the participants solid liquid gas the direction of the chemical reaction and the amount of each substance.
The word equation for this reaction would be X Y 2 XY The number of atoms of elements X and Y in the above-mentioned. Methods of balancing chemical equations 1. First write the unbalanced chemical equation.
Balanced chemical equations have the same number and type of each atom on both sides of the equation. Identify the reactants and the products in the reaction and write their chemical formulae. List each element and how many atoms it has underneath the reactants and products.
There is 1 atom in Fe. The next step for balancing the chemical equation is to determine how many atoms of each element are present on each side of the arrow. The products are on the right side.
Ca3P2 H2O CaOH2 PH3 Mins result. A chemical equation describes what happens in a chemical reaction. I First write the skeleton equation.
Or Easiest method for balancing the equation. Ca3P2 H2O Ca3OH2 PH3 Which best describes Mins method for balancing the chemical equation. Remember your reactants are on the left side of your equation.
Recognizing that chemical equations specify relative amounts of reactants and products. Ii The same total of charges should appear on the. Ratio reaction number amount.
There are 2 atoms of iron and 3 atoms of oxygen in Fe 2 O 3. Count the number of atoms of each element in the reactants and the products. To do this keep in mind a subscript indicates the number of atoms.
Add your answer and earn points. When balancing equations it is usually best to balance the elements that are present in the fewest molecules and then balance the elements that are present in more molecules. By putting a coefficient of 2 in front of 2HNO3 there are then 2 N atoms on both sides of the equation.
Browse more Topics under Chemical Reactions And Equations. The first step in balancing a chemical equation is to identify your reactants and your products. For this equation our reactants are Fe and O2.
Let us consider the formation of water from the combination of oxygen and hydrogen. To balance out the atoms add in coefficients. Mn 2 S 6.
Min is asked to balance the equation below Which best describes Mins method for balancing the chemical equation question 1 See answer passwod4548 is waiting for your help. Balancing redox equations ion-electron method First the general unbalanced reaction is placed in its ionic form. This method of balancing chemical equations involves assigning algebraic variables as stoichiometric coefficients to each species in the unbalanced chemical equation.
Thus we may take one of the coefficients to be 1 arbitrarily which then leaves only three unknowns and three. First draw boxes around each formula without changing anything inside the boxes. HCl Na 2 S H 2 S NaCl H 1 H 2 Cl 1 Cl 1 Na 2 Na 1 S 1 S 1 2.
The following sequential steps be taken to obtain a balanced chemical equation. For example O 2 has 2 atoms of oxygen. Shortcut for balancing the equation.
Finally the unknowns are determined by one of the algebraic methods of reduction equalization or substitution and the coefficients that result in the correctly balanced equation are obtained. For example reactants X and Y2 react to form a compound XY. For example H2 O2 H2O Caution.
Write down the oxidation numbers for each type of atom on both sides of the equation. Chemical Reactions and Equations. In order to better explain this method the reaction between glucose and oxygen.
KMnO 4 KI H2SO 4 I 2 MnSO 4. Fe O 2 Fe 2 O 3. Chemical equations are balanced for mass and.
Balancing an Equation The first step in balancing an equation is to count the number of atoms of each element on both sides of the equation. Steps to balance a chemical equation through inspection ESAED When balancing a chemical equation there are a number of steps that need to be followed. Then this equation is divided into two half-reactions the oxidation and.
This is a typical acid reaction. It is incorrect because the identity of the original product has changed. Do not change formula of any constituent while balancing the equation.
The reactant chemicals are listed on the left while the product chemicals are listed on the rightBecause atoms cannot be generated or destroyed in a chemical reaction. The coefficients in a balanced equation must be the simplest whole number ratio. These variables are used in mathematical equations and are solved to obtain the values of each stoichiometric coefficient.
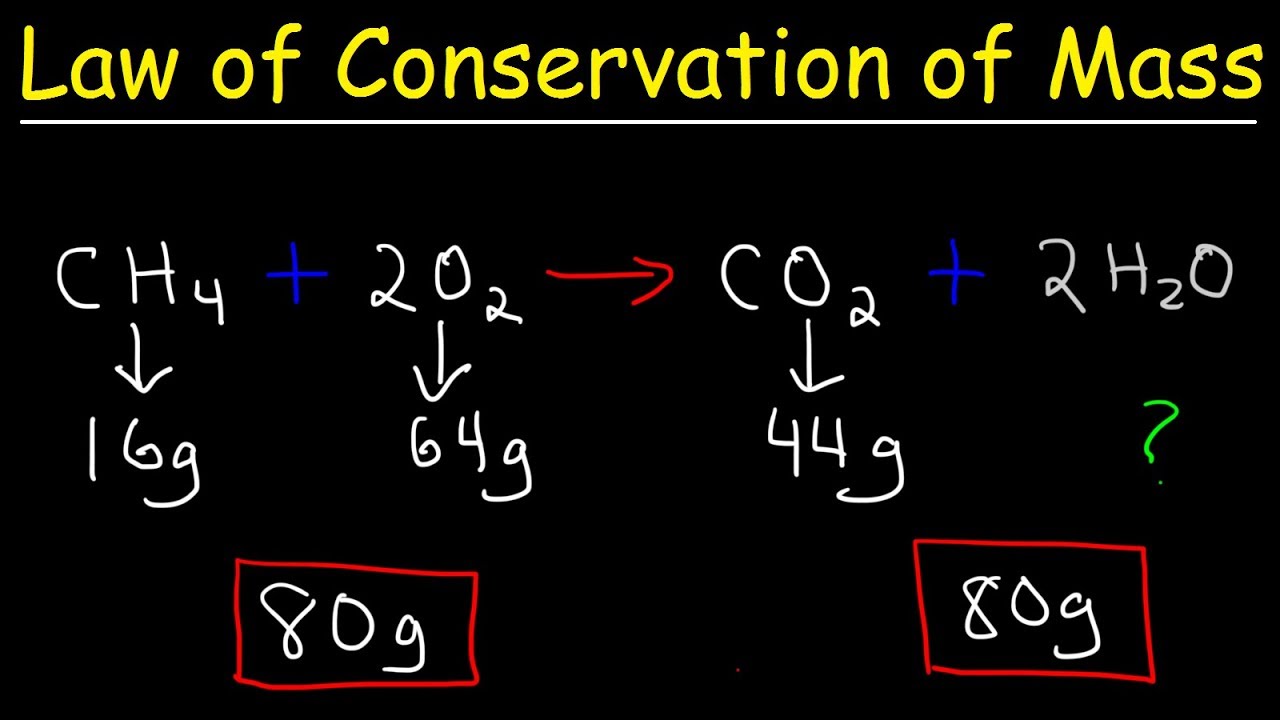
How Does The Process Of Balancing Equations Satisfy The Law Of Conservation Of Mass Lisbdnet Com

Balancing Chemical Equations With Substitution Chemistry Study Com
0 Comments